How do we make hydrogen from coal, and is it really a clean fuel?
Energy giant AGL this week unveiled plans to produce hydrogen power at its Loy Yang A coal station. But how do we transform coal, which is often thought of as simply made of carbon, into hydrogen – a completely different element?
What is coal made of?
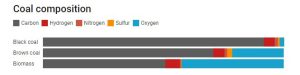
In simple terms, coal is a mixture of two components: carbon-based matter (the decayed remains of prehistoric vegetation) and mineral matter (which comes from the ground from which the coal is dug). The carbon-based matter is composed of five main elements: carbon, hydrogen, oxygen, nitrogen and sulfur.
You can think of coal’s formation process as a progression from biomass (newly dead plant matter) to charcoal (almost pure carbon). Over time, the oxygen and some hydrogen are gradually removed, leaving more and more carbon behind.
Brown coal thus contains slightly more hydrogen than black coal, although the biggest difference between the two is in their carbon and oxygen contents.
What is gasification?
We can understand gasification by first understanding combustion. Combustion, or burning, is the complete oxidation of a fuel such as coal, a process that produces heat and carbon dioxide. Carbon dioxide itself cannot be further oxidized, and thus is the non-combustible end product of the burning process.
In gasification, however, the coal is not completely oxidized. Instead, the coal is reacted with a compound called a gasification agent. Gasification is endothermic, which means it doesn’t produce heat. Quite the opposite, in fact – it needs heat input to progress. Because the resulting gas is not fully oxidized, that means it can itself be burned as a fuel.
So how do we make hydrogen?
Now we know the key concepts, let’s start again at the start. To produce hydrogen from coal, the process begins with partial oxidation, which means some air is added to the coal, which generates carbon dioxide gas through traditional combustion. Not enough is added, though, to completely burn the coal – only enough to make some heat for the gasification reaction. The partial oxidation also makes its own gasification agent, carbon dioxide.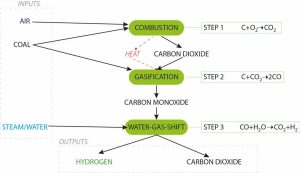
Carbon dioxide reacts with the rest of the carbon in the coal to form carbon monoxide (this is the endothermic gasification reaction, which needs heat input). No hydrogen yet.
Carbon monoxide in the gas stream is now further reacted with steam, generating hydrogen and carbon dioxide. Now we are making some hydrogen. The hydrogen can then be run through an on-site fuel cell to generate high-efficiency electricity, although the plan at Loy Yang A is to pressurise the hydrogen and ship it off to Japan for their Olympic showcase.
Brown coals are generally preferred for gasification over black coals for several reasons, which makes the brown coal of Victoria’s Latrobe Valley a good prospect for this process.
The main reason is that, because of the high oxygen content of this type of coal, it is less chemically stable and therefore easier to break apart during the gasification reaction. Plus there is a small boost from the hydrogen that is already present in the coal.
Hydrogen produced in this way is not a zero-emission fuel. Carbon dioxide is emitted through the combustion and thermal decomposition reactions, and is also a product of the reaction between carbon monoxide and water to make hydrogen and carbon dioxide.
